Patient Education
The treatment plan will be an evolution depending upon earlier interventions, surgeries, and imaging studies. Educating the patient to the understanding of the cause and treatment of the pain will always be the emphasis of the doctor patient relationship.
An understandable treatment plan will be tailored to the cause of the pain.
Injection therapy is directed under fluoroscopic guidance, and can range from epidural treatment, to facet injections, to major joint injections, to sympathetic blocks. Medications are also utilized with the emphasis on minimizing narcotic usage. Various medication classes are employed, and often times antidepressants, anticonvulsants, or compounded creams are prescribed.
If pain control is suboptimal, despite simpler options, more complex treatment plans are used. Patients may progress to implantable devices, including spinal cord stimulators or intrathecal programmable pumps.
The goal of treatment is to return to a functional lifestyle. This includes sleeping throughout the night, working, doing household choirs, and enjoying social activities.
Do you have SI Joint Pain?
The SI joint can be a significant cause of lower back pain. Clinical publications have identified the SI joint as a pain generator in 15-30% of chronic lower back pain patients.1-4 In addition, the SI joint is a pain generator in up to 43% of patients with continued or new onset lower back pain after a lumbar fusion.5
Sacroiliac Joint (SI Joint) Anatomy
The sacroiliac joint (SI joint) is located in the pelvis; it links the iliac bones (pelvis) to the sacrum (lowest part of the spine above the tailbone). It is an essential component for energy transfer between the legs and the torso.
SI Joint Fusion with iFuse TORQ® Implant System
Dr. Kloster is trained in the latest minimally invasive surgical (MIS) techniques, including use of the iFuse Implant System® from SI-BONE®, a medical device company pioneering MIS sacroiliac (SI) joint treatment. The iFuse Implant System is intended for SI joint fusion for some causes of SI joint pain. SI joint treatment using the patented triangular design of the iFuse implant has been clinically evaluated more than any other SI joint fusion procedure. More than 100, peer-reviewed publications demonstrate the safety, durable effectiveness, and biomechanical and economic benefits of the iFuse implant (www.si-bone.com/results.) The iFuse implant is the only SI joint fusion device with clinical studies, including two randomized controlled trials1,2, demonstrating that treatment improved pain, patient function, and quality of life.1-6 As with any minimally invasive surgical procedures, there are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit www.si-bone.com/risks.
References
- Polly DW, et al., and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10:Article 28. DOI: 10.14444/3028
- Dengler J, et al. Randomized Trial of Sacroiliac Joint Fusion vs. Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg Am. 2019;101(5):400-11. DOI: 10.2106/JBJS.18.00022.
- Duhon B, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T, on behalf of the SIFI Study Group. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter Trial. Int J Spine Surg. 2016;10:Article 13. DOI: 10.14444/3013
- Dengler J, et al. on behalf of the INSITE, iMIA and SIFI study groups. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint – a Pooled Analysis. Spine. 2017;42(21):1664-73. [Epub 2017 Mar 27]. DOI: 10.1097/BRS.0000000000002169
- Whang PG, et al. Long-Term Prospective Clinical and Radiographic Outcomes After Minimally Invasive Lateral Transiliac Sacroiliac Joint Fusion Using Triangular Titanium Implants. Med Devices (Auckl). 2019;12:411-422. DOI: 10.2147/MDER.S219862
- Patel V, et al. Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants: 24-Month Follow-Up. Med Devices (Auckl). 2021;14:211-216. DOI: 10.2147/MDER.S314828
Indications
The iFuse Implant System® is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. It is also intended for sacroiliac fusion to augment immobilization and stabilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion or for acute, non-acute, and non-traumatic fractures involving the sacroiliac joint. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit www.si-bone.com/risks
Find Out More
Where can I learn more about the iFuse Implant System®?
What is the Minuteman?
The Minuteman® is a minimally invasive, interspinous-interlaminar fusion device intended for the fixation and stabilization of the thoracic, lumbar, and sacral spine while awaiting bony fusion to occur The Minuteman® is designed for attachment to the posterior non-cervical spine at the spinous processes through its bilateral locking plates, and it is intended for use with bone graft material placed within the device. It provides immobilization and stabilization of the spinal segments. The core threaded post allows for optimal placement and a wide range of sizes allows for enhanced anatomical fit. The Minuteman® is delivered sterile and individually packed.
How is the Minuteman Procedure performed?
During the procedure, a 1-inch incision is made on the side of your body, dilation is used to access the spine, and the Minuteman® is implanted with bone graft material. The lateral approach does not require dissection or stripping of the sensitive back muscles, bones or nerves.This advantageous approach may lead to a shorter operative time, less blood loss, reduced hospital stay, and a faster recovery time. Watch our animation below.
How is the Minuteman different from traditional back surgery?
What are the potential benefits of the Minuteman procedure?
The Minuteman may provide the following benefits:
- Reduced procedure time
- Decreased blood loss
- Same day surgery
- 1-inch incision
- Little to no post-op pain
- Ability to place at L5-S1
- Minimal tissue disruption
- Supraspinous Ligament remains intact
Is the Minuteman procedure right for me?
The following questions will help determine if the Minuteman® System is right for you:
- Does your back or leg pain worsen with prolonged standing or walking?
- Does your back or leg pain improve while you are sitting?
- Does leaning forward improve your pain?
If you answered “Yes” to any of these questions, Ask if the Minuteman Procedure may be right for you.
Find Out More
Where can I learn more about the Minuteman procedure?
Injection Therapy
Many types of injections can be used to treat small or large regions ranging from epidural treatment, facet injections, to major joint injections.
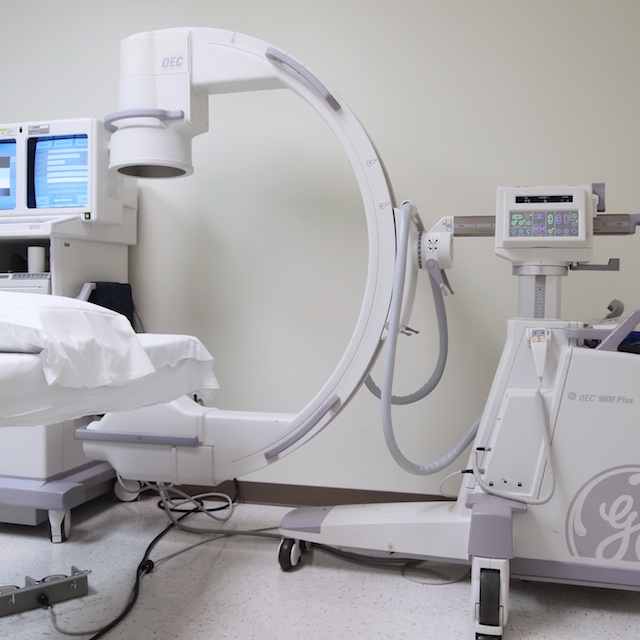
Surgical Imaging
Fluoroscopic Imaging allows a live view of many procedures ensuring a high level of precision when administering medications.
Device Implants
An intrathecal pump delivers small amounts of medication to a target area while spinal cord stimulators use small electric impulses.